Life sciences companies need specialized digital asset management platforms that surpass generic DAM capabilities to handle the regulatory requirements of pharmaceutical marketing.
- MLR workflow automation and 21 CFR Part 11 compliance features ensure content meets FDA validation requirements that generic systems can’t support.
- Global regulatory jurisdiction management automatically applies region-specific compliance requirements across different markets and regulatory bodies.
- AI-powered content detection and compliance checking identify potential violations before human reviewers evaluate promotional materials.
- Specialized platforms deliver measurable results like Boston Scientific’s 21% reduction in MLR cycle times while maintaining strict regulatory standards.
Generic DAM systems create dangerous compliance gaps that can result in costly regulatory violations and delayed product launches.
When pharma companies start shopping for digital asset management platforms, they realize pretty quickly that healthcare marketing plays by a completely different rulebook than, say, retail or consumer goods. While a retail brand might worry about brand consistency across channels, life sciences organizations face the additional challenge of navigating complex regulations that can make or break a product launch.
The pharmaceutical industry generates an enormous volume of digital content, from clinical trial documentation and regulatory submissions to promotional materials and educational resources for healthcare professionals. Managing these assets is about ensuring every piece of content meets stringent compliance requirements that vary by region, product type, and intended audience.
Industry data shows that pharmaceutical companies have paid over $126 billion in penalties since 2000 across 1,323 enforcement records. FDA violations typically range from $10,000 to $20,000 per violation. The cost of compliance is also operational, with delayed product launches, damaged brand reputation, and decreased market confidence.
This comprehensive guide explores what makes life sciences digital asset management fundamentally different from standard DAM solutions and why these differences matter for pharmaceutical organizations seeking to accelerate time-to-market while maintaining regulatory integrity.
Why Do Generic DAM Systems Fall Short for Life Sciences Companies?
Traditional DAM platforms excel at storing, organizing, and distributing digital content across marketing teams and creative agencies. They offer robust search capabilities, version control, and brand management tools that serve most industries well. However, pharmaceutical companies quickly encounter limitations that can create serious operational and compliance risks.
Regulatory Compliance Gaps in Standard DAM Platforms
Here’s where generic DAM systems hit a wall: they simply don’t have the specialized workflows pharma needs for content approval. Think about MLR reviews—these aren’t your typical ‘manager signs off and you’re done’ situations. They require carefully orchestrated approval chains where each stakeholder has to give their thumbs up before content can move forward. Standard platforms typically offer basic approval workflows, but they can’t accommodate the complex, multi-jurisdictional requirements that pharmaceutical promotional materials demand.
Consider a global pharmaceutical launch requiring content approval across the United States, European Union, and Japan. Each region has distinct regulatory requirements, including FDA guidelines in the U.S., EMA standards in Europe, and PMDA regulations in Japan. A generic DAM system can’t automatically route content through region-specific approval processes or ensure that regulatory disclaimers comply with local requirements.
Pharmaceutical content often requires specialized metadata that generic systems fail to capture. Regulatory submission dates, clinical trial references, indication-specific approvals, and expiration dates for promotional claims are critical data points that must be tracked and monitored throughout the content lifecycle.
The High Cost of Non-Compliance in Pharmaceutical Marketing
When non-compliant content slips through and hits the market, pharmaceutical companies don’t just face a slap on the wrist—they’re looking at immediate remediation costs, legal fees piling up, and sometimes having to pull products from the market entirely. Healthcare False Claims Act settlements alone totaled $1.7 billion in fiscal year 2022, with many violations traced to inadequate content management and approval processes.
Beyond direct financial consequences, compliance violations damage relationships with regulatory agencies, potentially leading to increased scrutiny for future submissions. Healthcare professionals lose trust in pharmaceutical brands that distribute inaccurate or non-compliant information, directly impacting prescribing patterns and market share.
Operational costs compound these challenges. Teams spend excessive time manually tracking content versions, searching for approved materials, and coordinating with legal and regulatory departments. Without specialized DAM for pharma, marketing teams often resort to email-based approval processes that create audit trail gaps and increase the likelihood of errors.
What Makes Life Sciences Digital Asset Management Different?
Life sciences digital asset management addresses the unique challenges pharmaceutical companies face by incorporating industry-specific features. These specialized capabilities transform content operations from a compliance bottleneck into a competitive advantage.
Medical, Legal, and Regulatory Workflow Automation
MLR workflows form the backbone of pharmaceutical content operations, ensuring that all promotional materials undergo appropriate review before distribution. Specialized DAM platforms automate these processes by creating configurable approval chains that route content based on asset type, intended audience, geographical market, and regulatory requirements.
Smart MLR automation does the heavy lifting by figuring out exactly who needs to review what. A piece of promotional content aimed at doctors? That goes through one approval sequence. Patient education materials? That’s a different path entirely. The system knows the difference and routes accordingly. The system automatically notifies reviewers when content requires their attention and tracks approval status in real time.
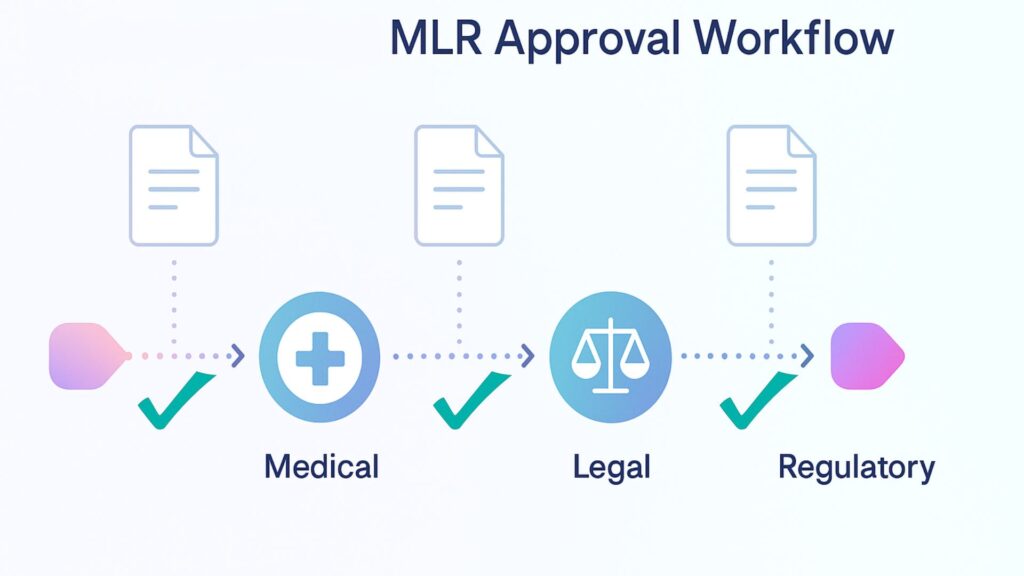
These platforms also support parallel review processes where multiple stakeholders can simultaneously evaluate different aspects of content. Medical reviewers focus on clinical accuracy, legal teams ensure compliance with advertising regulations, and regulatory specialists confirm adherence to submission requirements. This concurrent approach reduces approval times while maintaining thorough oversight.
21 CFR Part 11 Compliance and FDA Validation Requirements
The FDA’s 21 CFR Part 11 regulation establishes requirements for electronic records and signatures used in pharmaceutical manufacturing and clinical trials. While not all pharmaceutical marketing content falls under this regulation, many assets (particularly those related to clinical communications and regulatory submissions) require compliant management systems.
Specialized life sciences DAM platforms come equipped with the full compliance package: validated e-signatures, detailed audit trails that track every single action, and access controls tight enough to satisfy even the most scrutinous FDA inspector. These features ensure that content modifications are properly documented, reviewer identities are authenticated, and all system interactions generate immutable records suitable for regulatory inspection.
Validation documentation is another critical differentiator. Pharmaceutical companies must demonstrate that their content management systems operate according to predefined specifications and produce consistent, reliable results. Generic DAM platforms rarely provide the validation protocols and documentation packages that pharmaceutical quality assurance teams require.
Global Regulatory Jurisdiction Management
Pharmaceutical companies operate across multiple regulatory jurisdictions, each with distinct requirements for content approval, distribution, and archival. The European Medicines Agency enforces different promotional guidelines than the FDA, while emerging markets may have additional restrictions on pharmaceutical advertising and education.
Specialized DAM platforms manage these complexities by incorporating jurisdiction-specific business rules that automatically apply appropriate compliance requirements based on content destination and asset type. The system can prevent distributing content that lacks proper approvals for specific markets while ensuring that regional variations meet local regulatory standards.
This capability extends to automated disclaimer and fair balance requirements. Different markets mandate specific safety information, contraindication warnings, and prescribing information that must accompany promotional content. Advanced DAM systems automatically append appropriate disclosures based on content type and distribution channel, reducing manual errors and ensuring consistent compliance.
AI-Powered Content Detection and Compliance Checking
Today’s life sciences DAM platforms use AI as your first line of defense, catching potential compliance red flags before your human reviewers even lay eyes on the content. AI algorithms trained on pharmaceutical regulations can detect missing disclaimers, inappropriate claims, and content that may require additional regulatory scrutiny.
These systems also identify AI-generated content and flag it for appropriate review processes. As pharmaceutical companies increasingly incorporate artificial intelligence into content creation workflows, distinguishing between human-created and AI-generated materials becomes vital for regulatory compliance and intellectual property management.
Predictive analytics help pharmaceutical teams identify content gaps and optimization opportunities. By analyzing approval patterns, usage metrics, and regulatory feedback, these systems can suggest improvements to content strategies and highlight materials that may require updates to maintain compliance.
What Are the Essential Features Every DAM for Pharma Must Include?
If you’re evaluating DAM solutions for your pharmaceutical organization, don’t settle for a one-size-fits-all platform. You need industry-specific features built specifically to handle the unique headaches that come with regulated content management. Here are the five essential features that distinguish specialized platforms from generic alternatives:

1. Automated MLR Approval Workflows
Robust MLR workflow capabilities must support complex, multi-stage approval processes with configurable routing rules based on content type, market destination, and regulatory requirements. The system should automatically notify reviewers, track approval status, and maintain comprehensive audit trails that document all review activities and decisions.
2. Version Control with Audit Trails
Pharmaceutical content requires meticulous version control. Specialized DAM platforms maintain detailed audit trails showing who modified content, when changes occurred, and what specific alterations were made. This capability proves essential during regulatory inspections and compliance audits.
3. Rights Management and Expiration Tracking
Pharmaceutical promotional materials often have limited approval periods and specific usage restrictions. Advanced DAM systems automatically track content expiration dates, send renewal notifications, and prevent distribution of expired materials. Rights management capabilities ensure that content usage aligns with regulatory approvals and licensing agreements.
4. Multi-Factor Authentication and Security
Given the sensitive nature of pharmaceutical content, including proprietary clinical data and competitive information, security measures must exceed standard DAM platform capabilities. Multi-factor authentication, role-based access controls, and encryption protocols protect valuable intellectual property while maintaining regulatory compliance.

5. Integration with Pharma-Specific Tools
Life sciences organizations typically use specialized software for clinical trial management, regulatory submissions, and sales force automation. DAM platforms must integrate seamlessly with these tools to avoid creating operational silos that reduce efficiency and increase compliance risks.
How Does Life Sciences DAM Drive Measurable Business Results?
The return on investment from specialized digital asset compliance extends beyond risk mitigation. Organizations implementing these platforms consistently report improved operational efficiency, faster time-to-market, and enhanced global coordination that drives competitive advantage.
Faster Time-to-Market for Promotional Content
Boston Scientific’s implementation of specialized DAM resulted in streamlined global content operations with integrated MLR approvals and distribution capabilities. The company achieved a 21% decrease in MLR process cycle times while maintaining strict compliance standards across multiple therapeutic areas and geographic markets.
Real-time visibility into approval status enables marketing teams to identify bottlenecks and proactively address potential delays. Project managers can simultaneously track content progress across multiple approval chains, allowing for better resource allocation and deadline management.
Now multiply these efficiency gains across a large pharma organization churning out hundreds of promotional materials that all need review and approval. The time savings add up fast. Automated workflows eliminate manual handoffs between review stages, while intelligent routing ensures that content reaches appropriate stakeholders without delays.
Reduced Compliance Violations and Associated Costs
Specialized platforms provide automated compliance checking that identifies potential issues before content enters formal review processes. Standardized approval workflows ensure consistent application of regulatory requirements across all content types and market destinations.
Reduced compliance violations strengthen relationships with regulatory agencies, potentially accelerating future product approvals and reducing the likelihood of enhanced regulatory scrutiny. Healthcare professionals develop increased confidence in pharmaceutical brands that consistently deliver accurate, compliant information.
Improved Global Content Consistency
Multinational pharmaceutical companies struggle to maintain brand consistency while accommodating diverse regulatory requirements across markets. Specialized DAM platforms centralize approved content templates, brand guidelines, and regulatory disclaimers that local teams can access and customize for regional requirements.
This capability proves valuable for global product launches where consistent messaging must be adapted for local regulations. Marketing teams can leverage approved content frameworks while ensuring that regional variations meet specific market requirements and cultural preferences.
Regulated Content Storage: Beyond Basic File Management
Pharmaceutical organizations must maintain comprehensive records that support regulatory submissions, clinical communications, and post-market surveillance activities throughout extended retention periods.
Metadata Requirements for Regulatory Submissions
Regulatory submissions are serious business when it comes to metadata. You need detailed documentation covering everything from how content was created to who reviewed it and what they decided. And all of this has to be organized exactly how regulatory agencies want to see it, then kept accessible for however long they say you need to keep it.
Electronic Common Technical Document (eCTD) submissions exemplify the complexity of pharmaceutical metadata requirements. Content must be organized according to standardized formats that facilitate regulatory review while maintaining complete audit trails that document all submission activities.
Specialized life sciences digital asset management platforms automatically capture and organize this metadata during content creation and review processes. Rather than requiring manual data entry, these systems extract relevant information from workflow activities and format it according to regulatory specifications.
Long-term Archival and Retrieval Systems
Pharmaceutical companies must retain promotional materials, clinical communications, and regulatory correspondence for extended periods that often exceed standard DAM archival capabilities. Some categories of content require permanent retention with guaranteed accessibility for regulatory inspection purposes.
Regulated content storage systems incorporate archival technologies that ensure long-term data integrity while maintaining retrieval capabilities. These platforms often utilize write-once, read-many (WORM) storage technologies that prevent unauthorized modification while preserving content accessibility.
Cloud-based archival solutions offer scalability for organizations managing large volumes of regulated content. However, pharmaceutical companies must ensure that cloud providers offer appropriate security certifications and compliance capabilities that meet industry requirements.
Implementation Considerations for Life Sciences Organizations
Deploying specialized DAM platforms in highly regulated environments requires careful planning that addresses both technical requirements and organizational change management. Pharmaceutical companies must balance operational efficiency gains with stringent compliance requirements throughout implementation processes.
Change Management in Highly Regulated Environments
Pharmaceutical organizations often maintain established processes that have been validated according to regulatory requirements. Implementing new DAM capabilities requires comprehensive change management that addresses both technical system migration and updated standard operating procedures.
Validation protocols must be established before system deployment to ensure that new DAM capabilities meet regulatory requirements and produce consistent, reliable results. These protocols typically include installation qualification, operational qualification, and performance qualification activities that document system compliance.
User acceptance testing in pharmaceutical environments includes compliance scenario validation. Teams must demonstrate that new workflows maintain regulatory compliance while improving operational efficiency across various content types and approval processes.
Training Teams on Compliance-First Content Operations
Pharmaceutical marketing teams require specialized training that emphasizes compliance considerations alongside operational efficiency. Training programs must address regulatory requirements, approval workflow procedures, and system capabilities that support compliant content management.
Role-based training makes sense because let’s face it—your marketing managers don’t need to know everything your compliance team does, and vice versa. Everyone gets trained on exactly what they need for their part of the process. Marketing managers need comprehensive workflow management training, while content creators focus on asset preparation and metadata requirements. Compliance teams require detailed audit trail and reporting capabilities instruction.
Ongoing training programs address regulatory updates and system enhancements that impact content operations. Pharmaceutical regulations evolve continuously, requiring periodic training updates that ensure teams maintain current compliance knowledge and system proficiency.
Frequently Asked Questions
What is 21 CFR Part 11 compliance, and why does it matter for DAM? 21 CFR Part 11 is an FDA regulation that defines the criteria for electronic records and electronic signatures used in pharmaceutical operations. For DAM systems, this means implementing validated electronic signature capabilities, comprehensive audit trails, and access controls that meet FDA requirements. While not all pharmaceutical marketing content requires 21 CFR Part 11 compliance, materials related to clinical communications and regulatory submissions often do.
How does MLR workflow automation reduce approval times? MLR workflow automation eliminates manual handoffs between review stages and enables parallel review processes where multiple stakeholders can simultaneously evaluate different aspects of content. Intelligent routing automatically identifies which reviewers must evaluate specific content types, while real-time tracking provides visibility into approval status.
What security features are essential for a pharmaceutical DAM? Pharmaceutical DAM systems require multi-factor authentication, role-based access controls, encryption for data in transit and at rest, comprehensive audit trails, and integration with corporate identity management systems. Additional security considerations include geographic data residency requirements, SOC 2 Type II certification, and validation documentation that supports regulatory compliance audits.
How does life sciences DAM integrate with existing regulatory systems? Specialized pharmaceutical DAM platforms offer pre-built integrations with common regulatory tools, including Veeva Vault, clinical trial management systems, and regulatory submission platforms. These integrations enable seamless data exchange while maintaining audit trails and compliance controls. API capabilities support custom integrations with proprietary systems that pharmaceutical companies may use for specialized regulatory processes.
Transforming Life Sciences Content Operations
Life sciences digital asset management addresses the unique challenges pharmaceutical companies face. These specialized systems transform compliance from an operational bottleneck into a competitive advantage that accelerates time-to-market while maintaining rigorous regulatory standards.Aprimo’s specialized life sciences platform offers the compliance-first features and regulatory expertise that pharmaceutical organizations need to accelerate growth while maintaining the highest standards of compliance. Book a demo today to see digital asset management for life sciences in action.


