Navigate Regulatory Challenges with Aprimo’s Content Lifecycle Management
Reduce time-to-market while supporting the full content lifecycle from planning to MLR review. Securely manage and distribute approved content, all while remaining compliant.
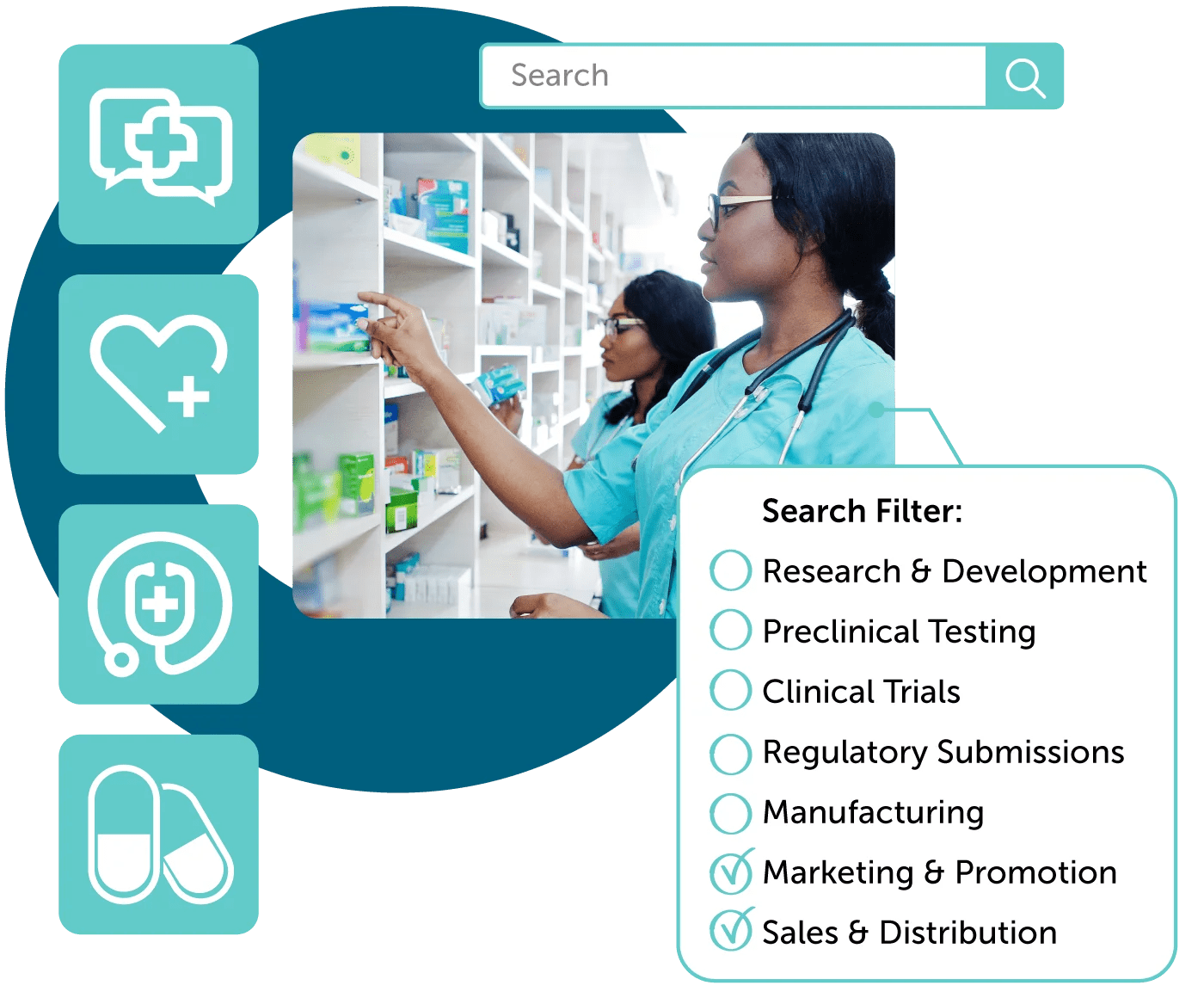





Cross-Functional Collaboration with Compliancy
Ensure compliancy of regulatory review (MLR) processes with automation. Simplified reference linking and tagging of promotional marketing materials such as text, PDF, and no-file content ensure all content is substantiated.
Centralized and Secure
Create integrated digital experiences that are always on-brand and compliant. Plan, manage, and prioritize all content and campaigns with agency and external partners to ensure brand integrity and consistency.

Streamlined Content Management to Reduce MLR Process Time
Boston Scientific utilizes Aprimo for content creation, MLR approvals, and content distribution globally. Insight into content creation and management activities is provided through Power BI reports, leading to streamlined processes and, a 21% decrease in MLR process cycle times and increased content reuse.

Stacey Hunsdon
Program Manager, MUFG Union Bank
Aprimo for the win!
“We use Aprimo as our Marketing project management tool as well as DAM. We had 2 different tools before and were not aligned correctly. We were able to build out our requirements with the Aprimo team to suit our exact needs. We are also able to get things changed as needed in our custom built version.”

Mihir Shah
Marketing & Advertising Technology Leader, Grainger
Grainger Embraces Digital Tools To Streamline Marketing
“It’s a fully automated, well-oiled integration where we hardly get any errors. We work very closely with Aprimo customer service and the consulting team, but it was a good effort that’s paying dividends for us now.”
Futureproofed Marketing Operations
Enact agile strategy pivots and rapid innovations with rich analytics and insights into how your content is being developed and where it is performing well. Robust usage trails and reports ensure all marketing activities meet regulatory requirements.
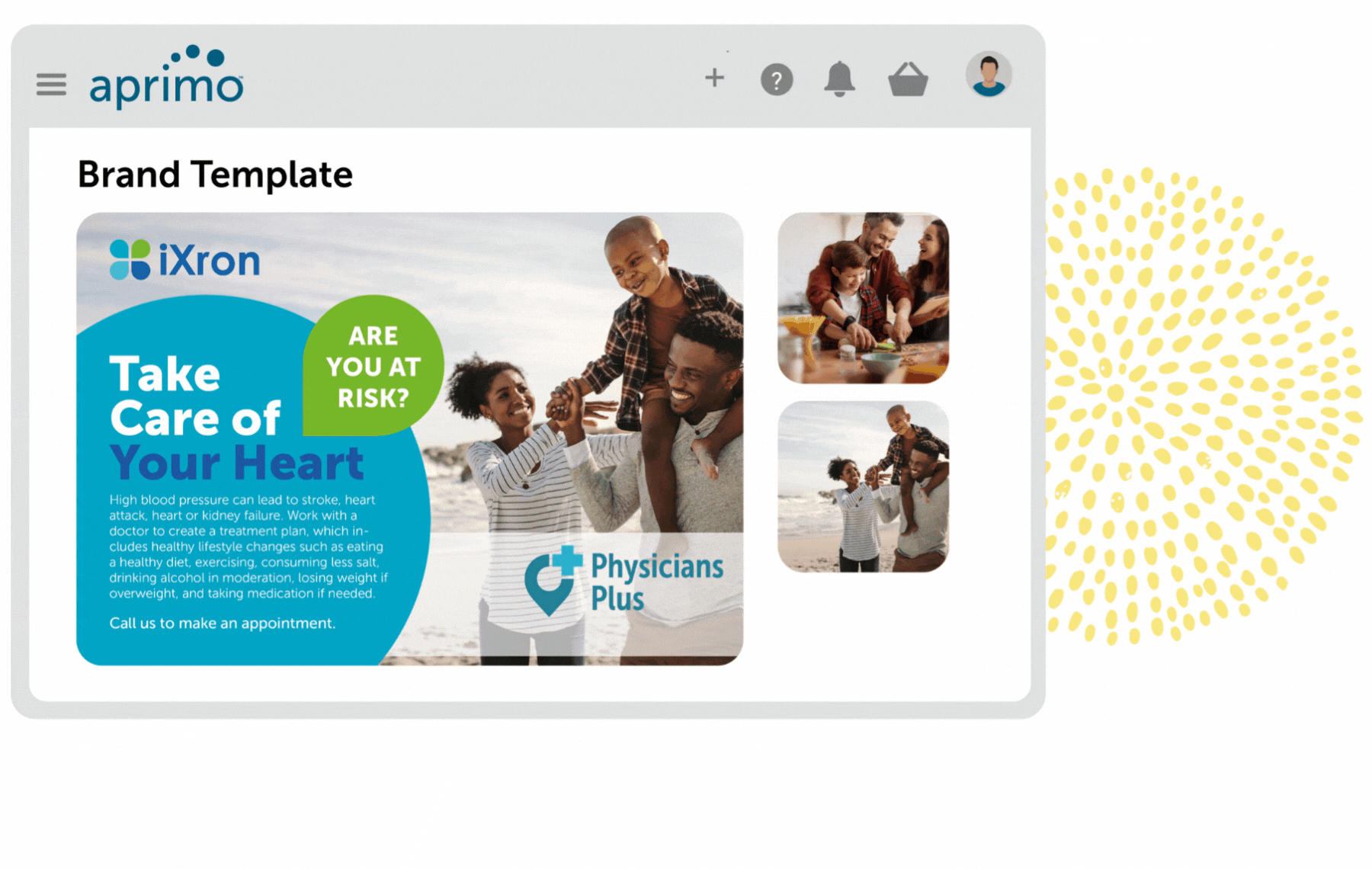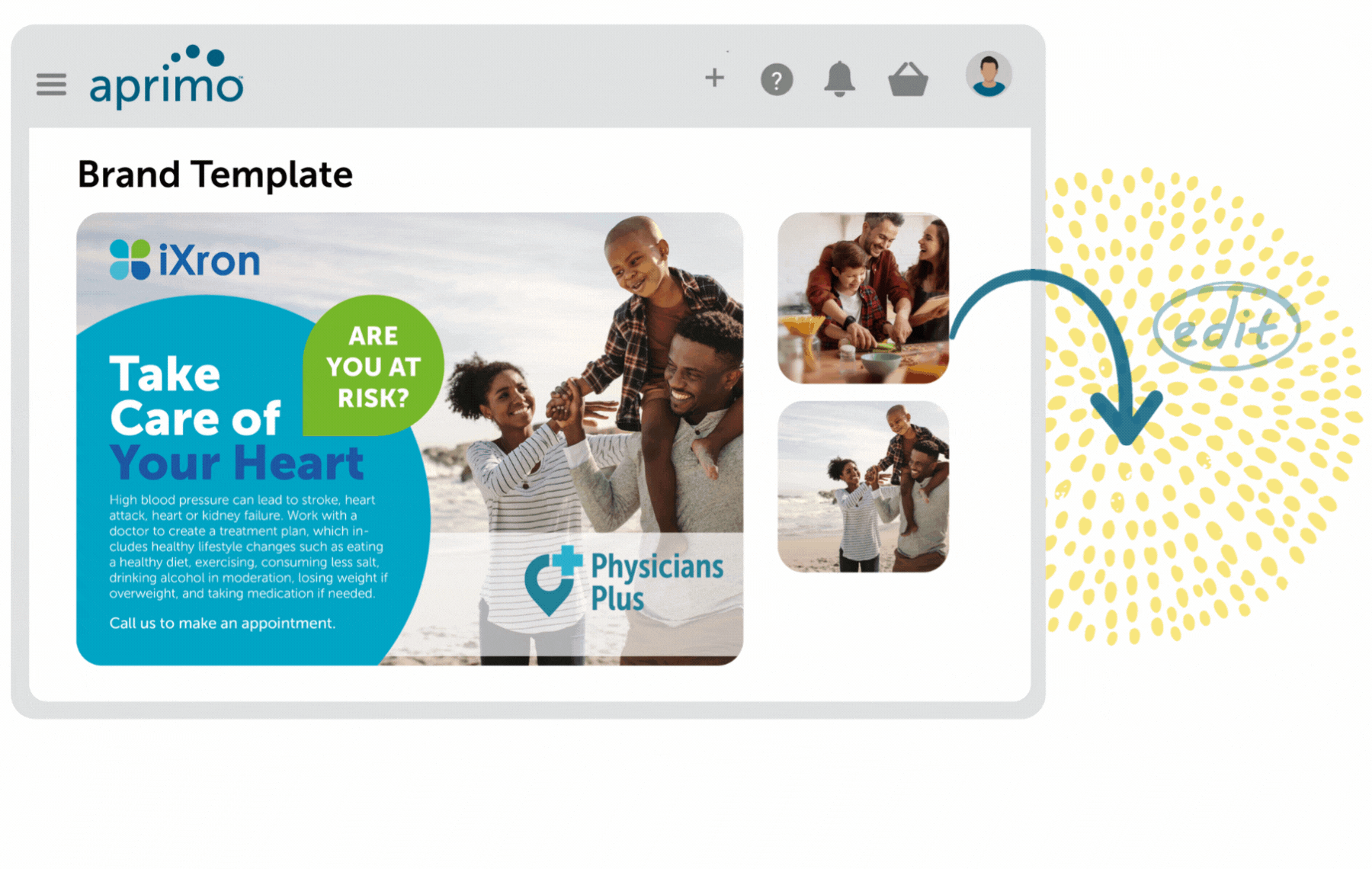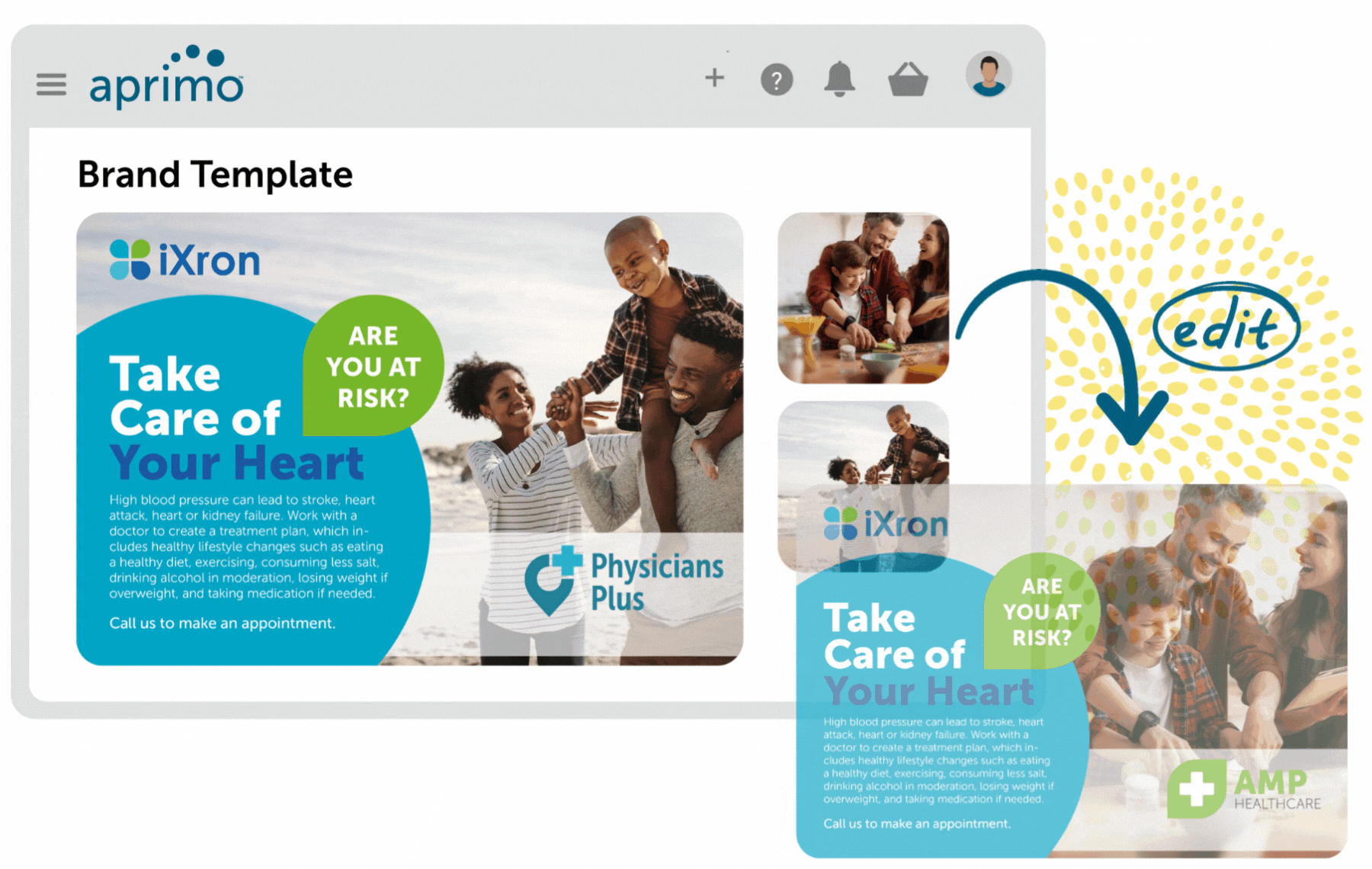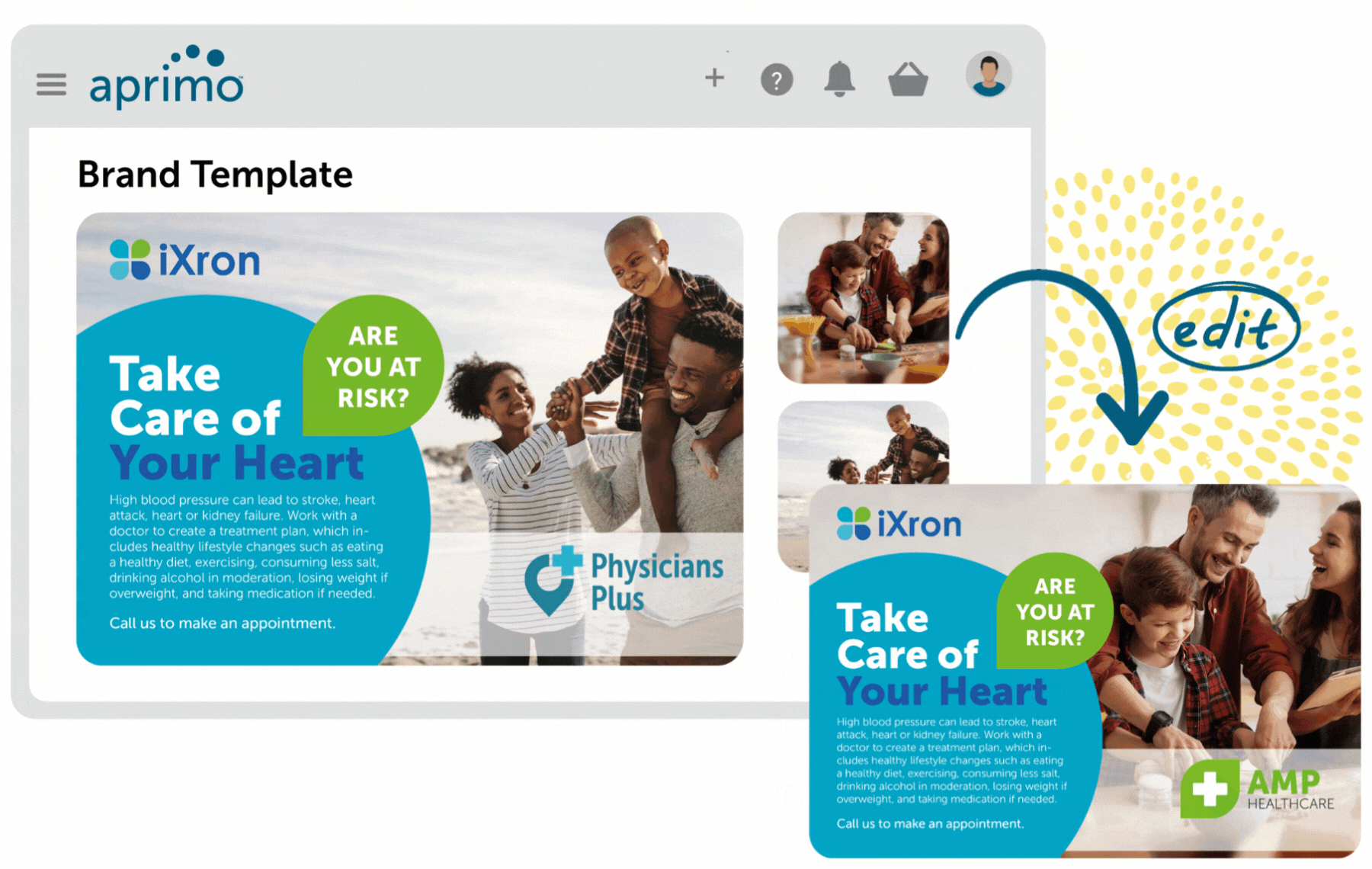
Key Benefits

Fully Compliant Regulatory Review and Workflows
Remain compliant with governing bodies and standard operating procedures (SOPs) with auditable, flexible template-based workflows that are secured by user rights.

Reference Linking and Audit Trails
Ensure claims in promotional materials are supported and approved for use for audit and compliance needs.

Fully Compliant with 21 CFR Part 11
Leverage secure compliance of two-factor authentication with secondary pin authentication when voting on an asset for reviews and approvals.

Robust Digital Rights Management
Reduce risk of contractual violations and regulatory fines by tracking use of promotional materials with user roles and workflow security that control what a user is entitled to do or see.

Record Retention and Purge Capabilities
Adhere to regulatory requirements of retaining records related to production and review of promotional materials with secure preservation of all records and activity.
Check related integrations

FORM 2253 Generation
by Aprimo
Automatically generate an FDA 2253 form and package for submission.

Compliance Attestation Form
by Aprimo
Easily generate and store attestation for compliance.

Indegene Connector for Aprimo DAM
by Indegene
mproved content searchability using pharma-specific tags, making it easier to find and reuse assets in Aprimo DAM with enriched metadata harvested from Indegene to create personalized customer experiences at scale.





